Therapeutic and diagnostic products
Femasys has achieved global regulatory approvals for a first-line infertility treatment and complimentary diagnostic products.
Infertility
Infertility has become a significant global problem, which may be the result of limited advancements over the last 30 years.
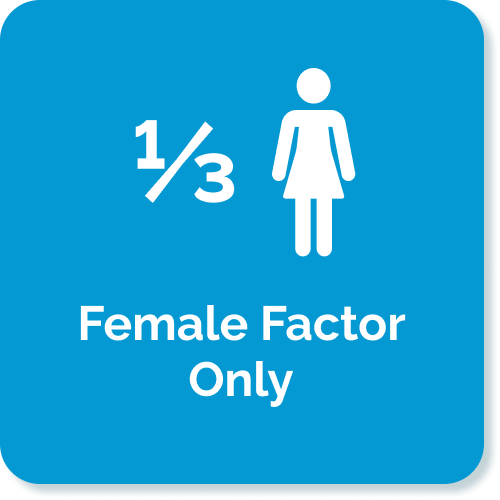
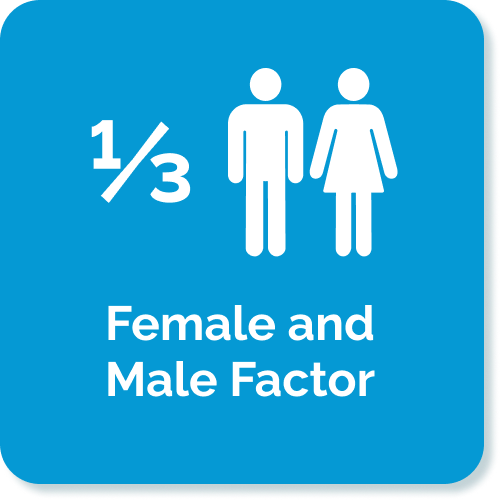
- Kumar N, et al. (2015) Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum Reprod Sci. 8(4): 191–196. doi: 10.4103/0974-1208.170370:
- Levine H, et al. (2023) Temporal trends in sperm count: a systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Human Reproduction Update, Vol.29, No.2, pp. 157–176, 2023
Our Infertility Portfolio
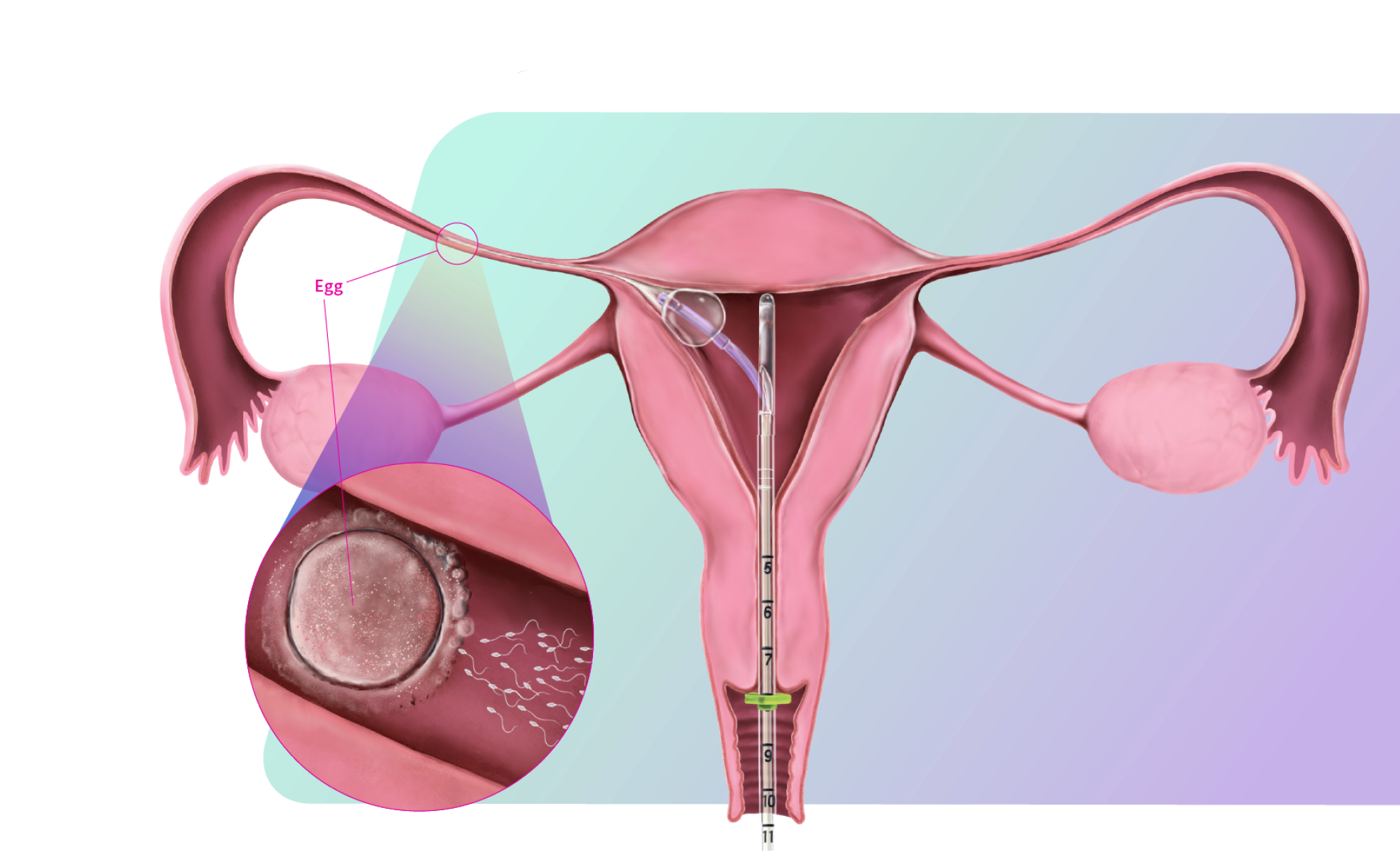
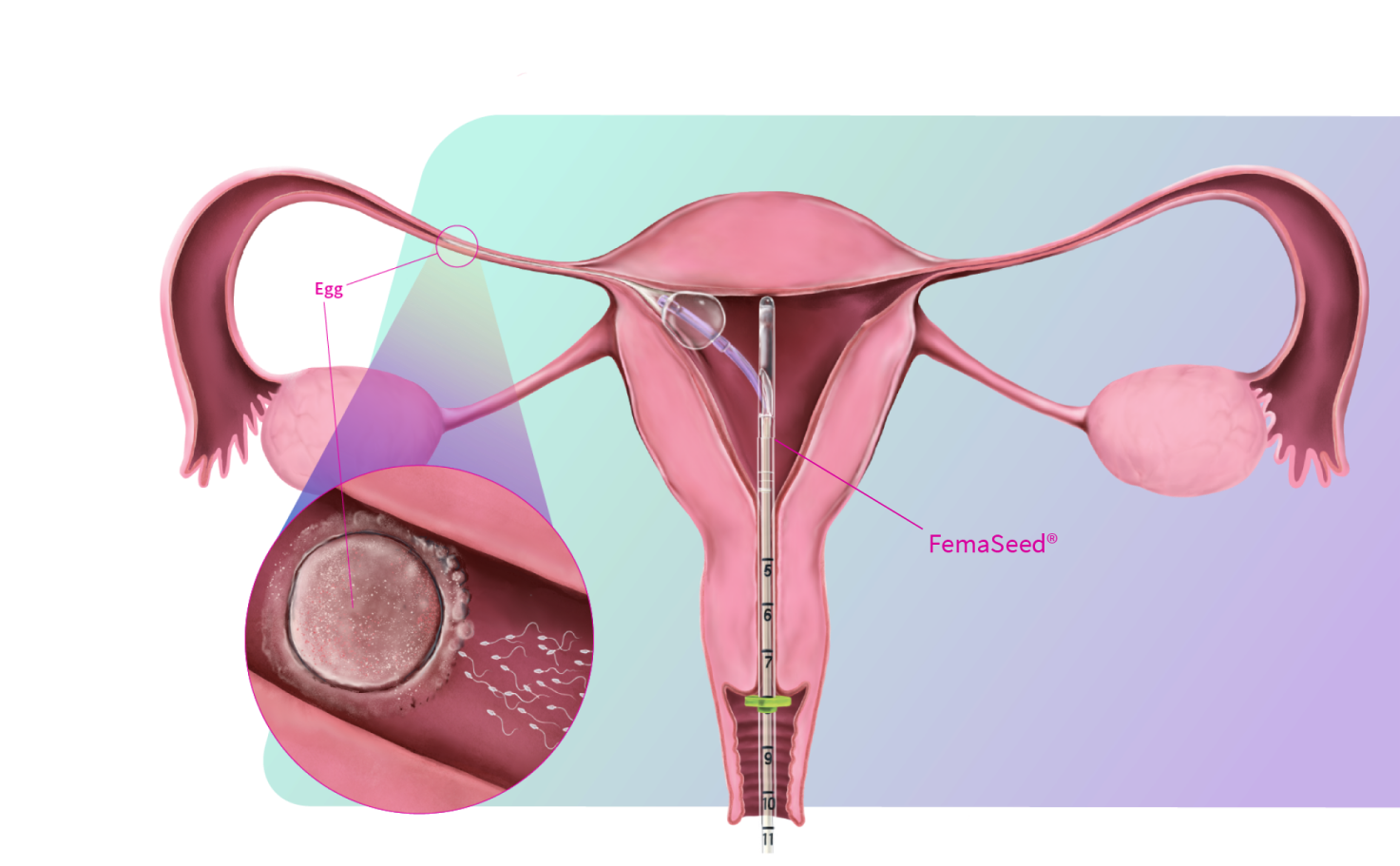
FemaSeed is the latest in artificial insemination technology, engineered to precisely deliver sperm into the fallopian tube, the site of conception, to enhance natural fertilization. As a highly cost-effective approach that carries notably reduced risks compared to in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), FemaSeed is positioned as a first-line therapeutic option.
FemaSeed has regulatory approvals in the U.S., Europe and Canada.

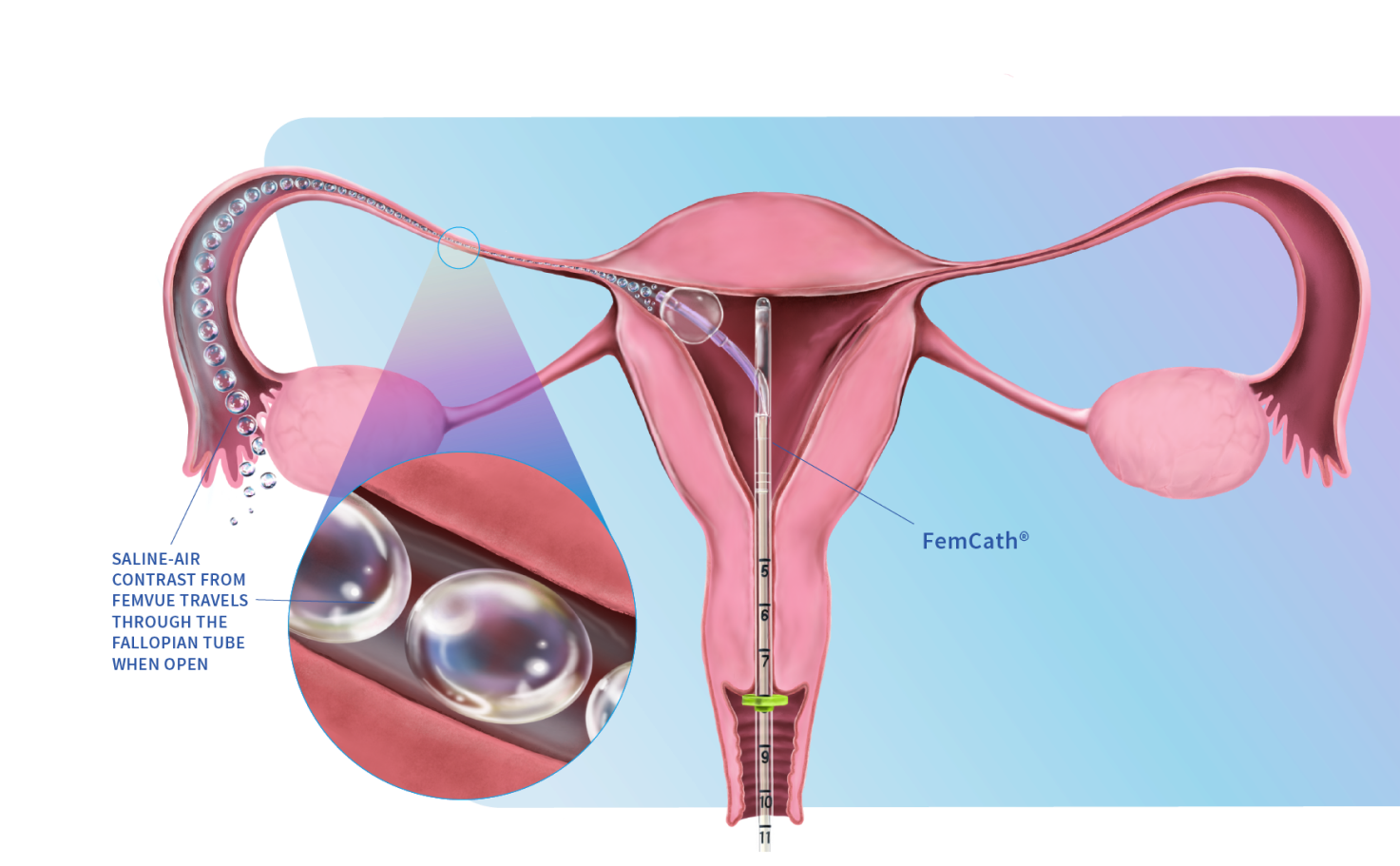
FemVue is the first FDA-cleared product that creates natural saline and air contrast and enables safe, reliable, and real time evaluation of the fallopian tubes with ultrasound. When performed with a uterine cavity assessment, a more comprehensive exam can be achieved from the comfort of the GYN’s office.
FemVue has regulatory approvals in the U.S., Europe, Japan and Canada.

FemCath, an FDA-cleared intrauterine catheter, is the first to allow for selective evaluation of a fallopian tube with contrast. FemCath features our proprietary delivery platform, which places balloon technology close to the opening of a selected fallopian tube for directed delivery.
FemCath has regulatory approvals in the U.S., Europe and Canada.